
It’s been a while since you’ve last ridden your two-stroke ski – be it a few weeks or several years – you’ve got the better part of the day off and you’re thinking it’s a good time to hit the water. With a gas can topped off with a fresh batch of premix you’re ready to hit the lake. But something just isn’t right. Sure, it fires up but man, your ski is running rough. No matter how much throttle you give it, that engine is running like crap. So, you pull the hood and the air filters and give the carburetors a look-see – and there it is: white stuff. A fine white film invading all of the tight crevices and edges inside and out of your carb. “What the heck is this?” you question. Thankfully, there’s a pretty simple answer: aluminum oxide.
Just as steel will corrode when exposed to moisture and the elements, so does aluminum also corrode – just in a very unique way. Conventional rust is formed by the reaction of iron and oxygen in the presence of water (or air moisture). Given enough time, oxygen and water will eventually convert any iron (or steel) entirely to rust and disintegrate. Unlike steel, which produces a flaky, fibrous layer commonly known as “surface rust,” aluminum naturally produces aluminum oxide, which for this example, is often found as a very fine powdery film gathering in corners, fissures and porous surfaces where moisture collects. Interestingly, aluminum oxide is produced by aluminum as a natural defense against further corrosion, acting as a protective layer against further exposure.
[As a side note, it is possible for components inside the carburetor to truly rust; this is because these items are made from inferior metals (ie. pot metal, untreated or raw metals, etc.), such as screws, plates and fittings that hold the carburetor’s internal components together. When rust (iron oxide) comes into contact with aluminum oxide, it can produce a “dingy, yellowish” color. –Ed.]
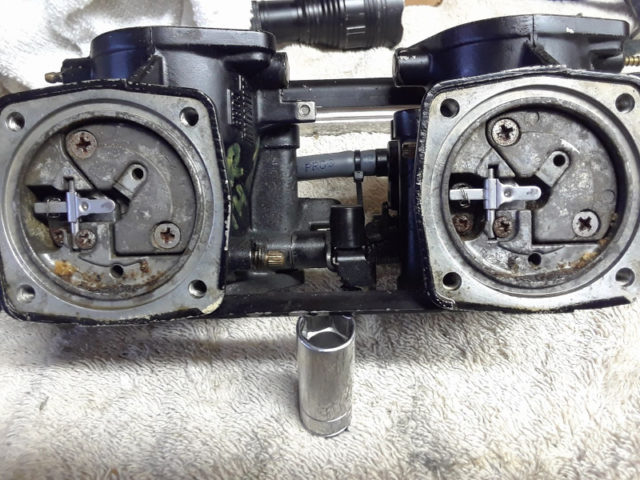
The aluminum oxide layer in the carburetor is formed as aluminum is exposed to water and oxygen, yes, but is also exacerbated when heat is applied. While organic and formic acids can also radically stimulate the formation of aluminum oxide, it’s also of great interest to note that alcohols can similarly corrode – particularly ethanol. In an article written by David Fuller, he writes, “ethanol is hygroscopic, which means it absorbs water. Ethanol-blended fuel will naturally hold 0.5 percent water in suspension, but once the water content exceeds this percentage, the water/ethanol mix becomes heavier than the gasoline portion of the fuel. This leads to what experts call ‘phase separation,’ which is the point at which the water/ethanol mix drops out of suspension and sinks to the bottom of the fuel tank.”
In the same article, he quotes Scott Diehl at Driven Racing Oil, who explains, “Ethanol in a modern fuel-injected [engine] is typically not a problem. The components used in these engines are more compatible, but carburetors are typically made from alloys that are more susceptible to corrosion–zinc, aluminum, and brass.” So what happens inside of your jet ski’s engine as the heat temperatures begin to rise? At first the water-heavy fuel will start to evaporate, leaving corrosive moisture-rich oxygen behind. Almost immediately, the aluminum begins to produce microscopic crystals, which when gathered in a moisture-rich environment – say inside the fuel delivery metering circuitry and fuel pump – looks almost like a white paste. As heat continues to cook the chemical reaction and evaporate the liquids in the carburetors, the white paste-like substance starts to dry into a fine, thin powder.
While trying to keep your carburetor from naturally producing aluminum oxide is all but impossible, there are ways to inhibit its growth: The first being the most simple; don’t let your watercraft sit for a period of time with water inside of the carburetors. Second, avoid using ethanol-rich blended fuels if possible. If “pure fuel” isn’t available to you, use a fuel stabilizer or fuel conditioner to prevent rust and corrosion associated with the use of ethanol fuels. It’s also heavily recommended to apply some fogging oil to the engine between lapses in use to coat surfaces prone to corrosion. And there’s something to be said for giving your engine a good spray down of a water-resistant mineral oil (ie. WD-40) between uses. Lastly, we recommend storing your ski indoors, in a dry, stable environment.
So there it is, some basic understanding of why you might be seeing some white, chalky or fine powdery film inside and around your carburetor. No, you didn’t suck up a bunch of white sand into your engine – and if caught early, isn’t a death sentence for your engine either. It’s just naturally-forming aluminum oxide and can be cleaned up pretty quickly. Sure, it ain’t pretty but it’s not catastrophic. Just take a little better care of your stuff and you won’t see it much in the future. These are hard, indisputable metallurgical facts, and any loud-mouthed, hairy-knuckled gorilla who tells you otherwise has no place near a set of tools.











